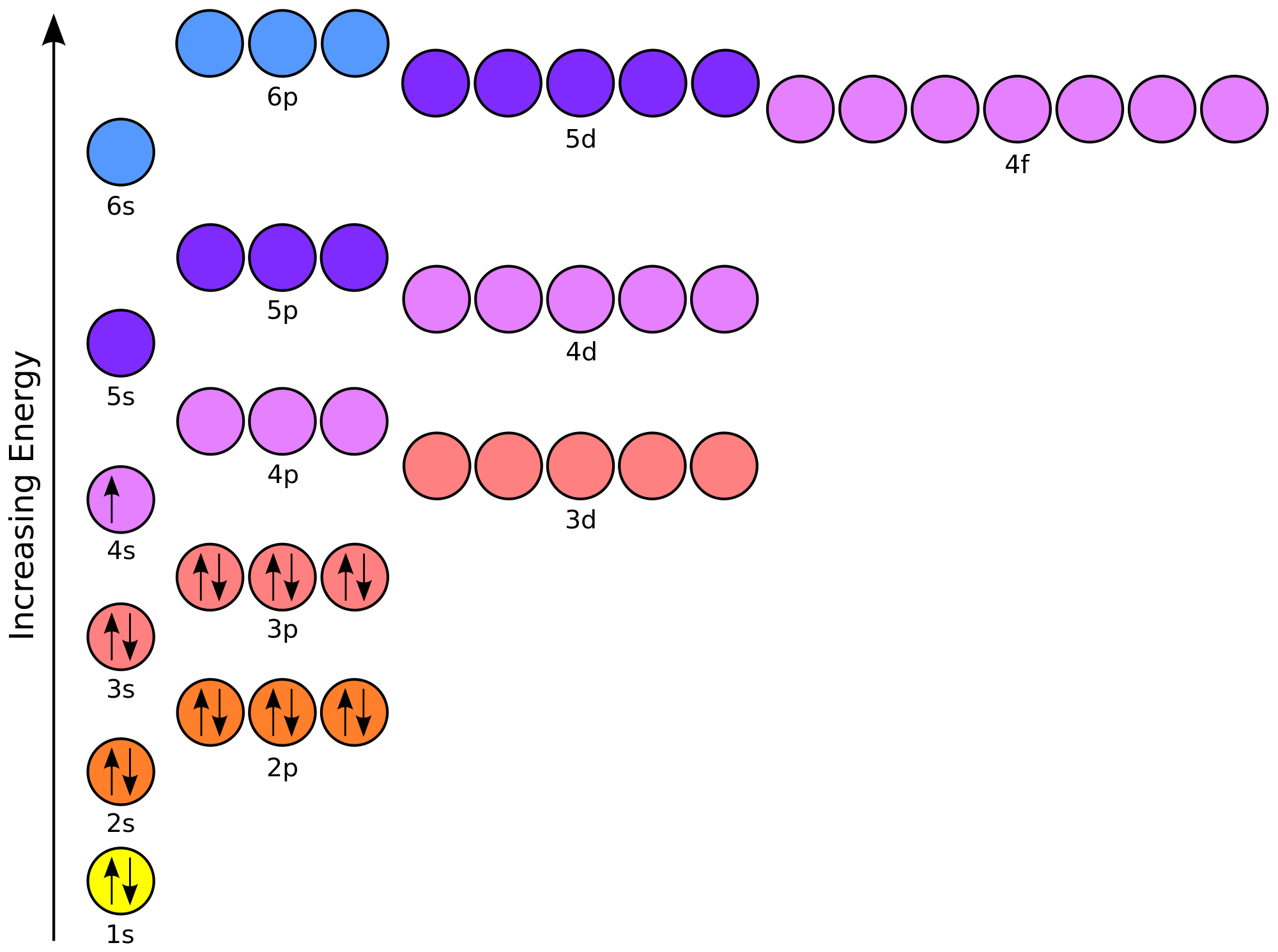
8+ orbital diagram of tin
729 gcm 3. Free Gift for you.
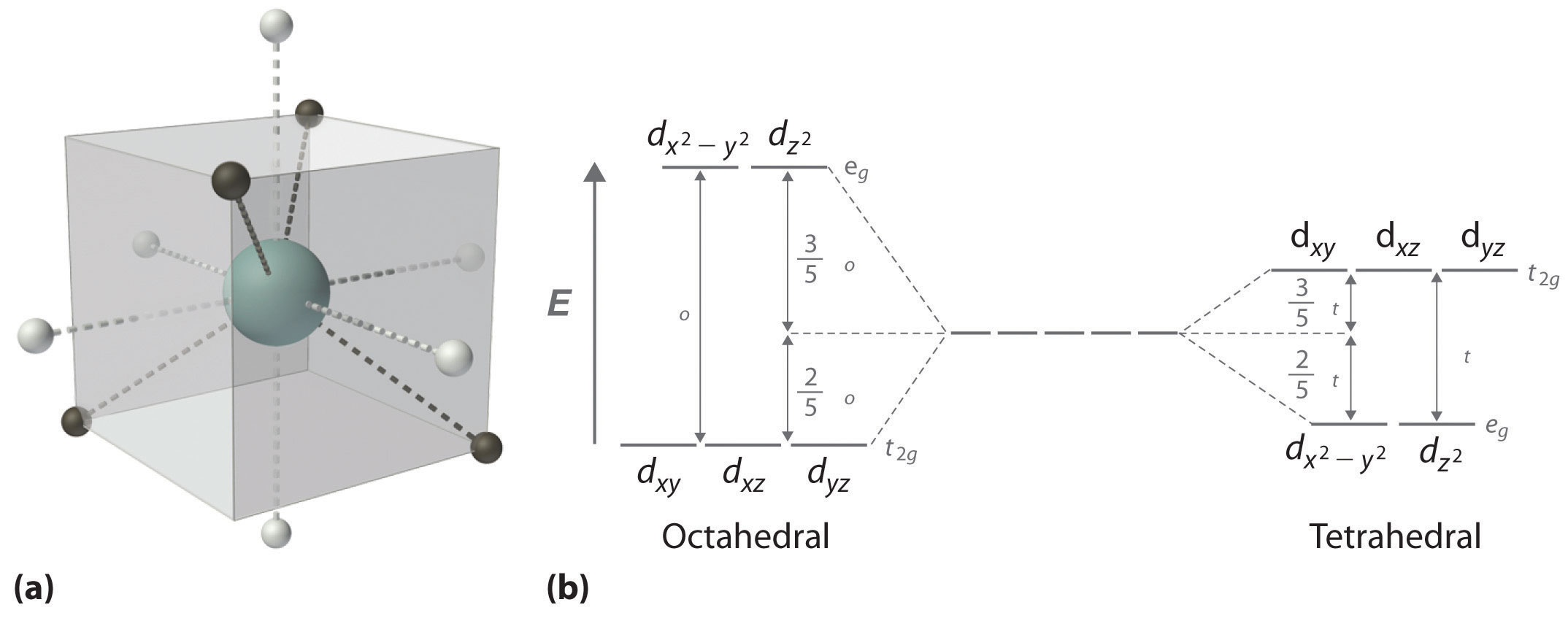
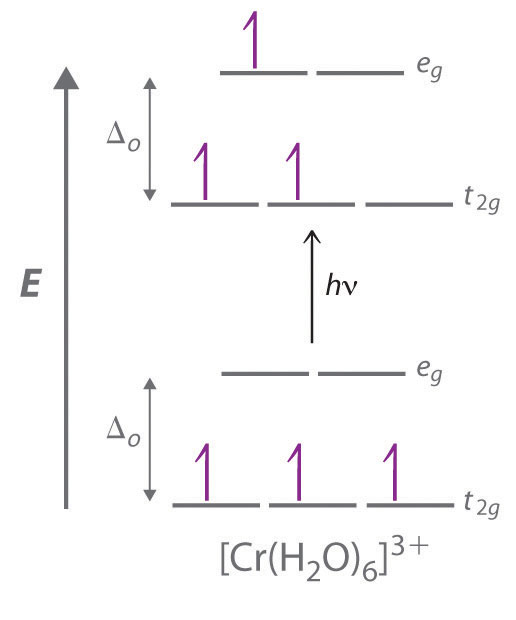
The D Block Elements
4 From its orbital.
. 3 From its bohr model. Orbital diagram for tin Orbit the path of a celestial body or other object in space governed by the gravitational attraction of other bodies. 2 in the five p one electron can be promoted giving us four our half filled orbitals which can.
The orbital diagram of tin shows that the 1s subshell has 2 electrons the 2s subshell has 2 electrons the 2p subshell has 6 electrons the 3s subshell has 2 electrons the. Orbital diagram of Sodium Na 12. So the next two electrons will enter the 4s orbital and the remaining two electrons will enter the 3d orbital.
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2. 2 8 18 18 4. 1s 2s.
Therefore the titanium full electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d. Electron configuration of Tin Sn Kr 4d 10 5s 2 5p 2. Electronic configuration of the Tin atom in.
Orbital diagram of Oxygen O 9. Orbital diagram of Fluorine F 10. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
In addition to listing the. Orbital diagram of Tin Sn 51. Sn Tin is an element with position number 50 in the periodic table.
Orbital diagrams Orbital box diagrams of all elements are mentioned in the chart given below. We need to first look at the atomic orbitals of 10 Tin contains four valence electrons to in the five s. Located in the V period.
The orbital diagram will be filled in the same order as described by the Aufbau principle. An orbit as the term is commonly used is the relative. Orbital diagrams Orbital box diagrams of all elements are mentioned in the.
2 Using periodic table. We can write the electron configuration of tin using four different methods. Orbital diagram of Neon Ne 11.
Orbital Diagrams Many times it is necessary to see all the quantum numbers in an electron configuration this the purpose of the orbital diagram. 1 Using aufbau principle. Answer 1 of 3.
18 32 32 18 8. The order in which the orbitals are filled with electrons from lower energy to higher energy is.
Question 26b0e Socratic
Electronic Structure And Bonding In Endohedral Zintl Clusters Chemical Society Reviews Rsc Publishing Doi 10 1039 D1cs00775k

Tuning Organic Room Temperature Phosphorescence Through The Confinement Effect Of Inorganic Micro Nanostructures Huang 2021 Small Structures Wiley Online Library

Ealom General Chemistry Pdf Pdf Ion Atomic Mass Unit
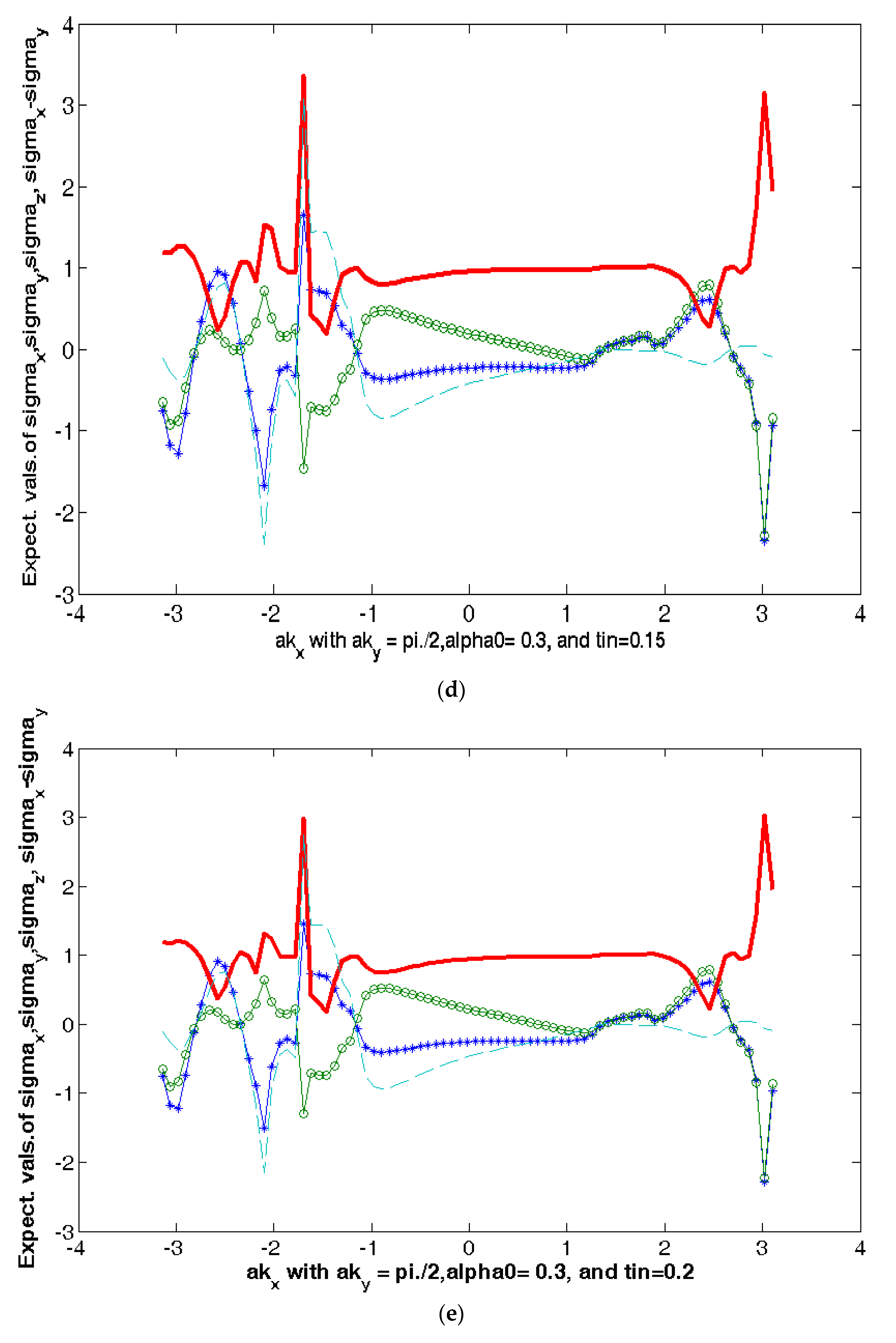
Symmetry Free Full Text Fledgling Quantum Spin Hall Effect In Pseudo Gap Phase Of Bi2212 Html
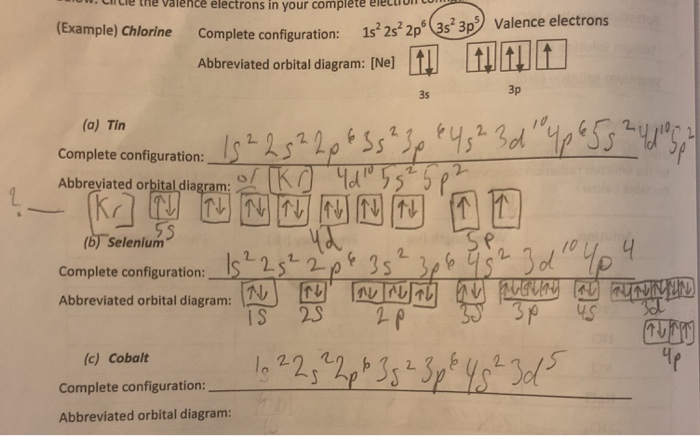
Solved I M Confused On How To Draw The Abbreviated Orbital Chegg Com

Tin Sn
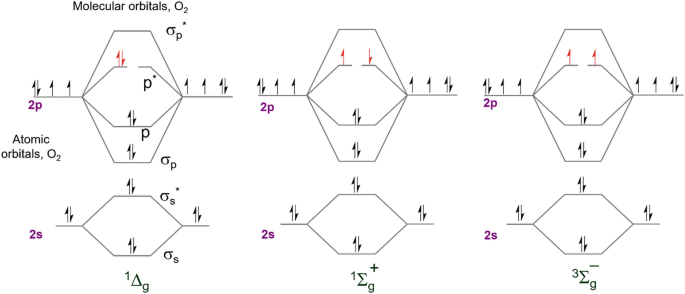
Photophysicochemical Processes Directed Within Nano Containers Springerlink

Webelements Periodic Table Osmium Properties Of Free Atoms
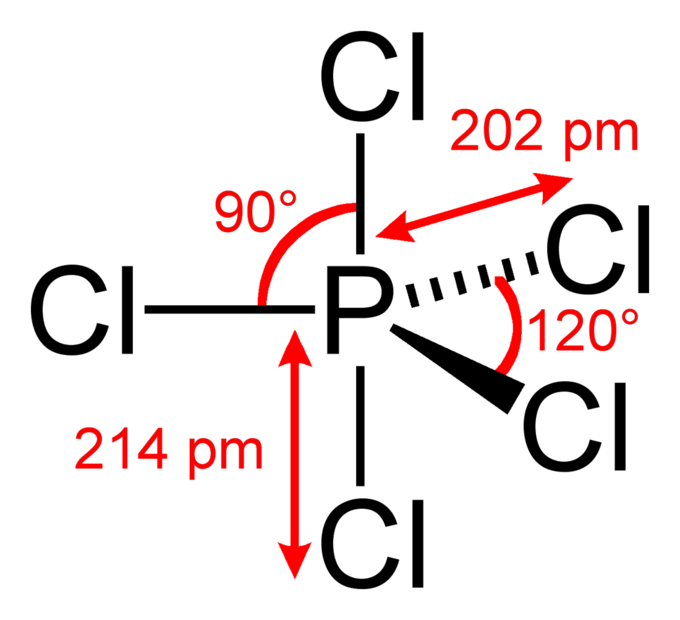
The Expanded Octet Introduction To Chemistry Course Hero

An Overview Of The Role Of Supramolecular Interactions In Gas Storage Using Mofs Sciencedirect

Sn Tin Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids
The D Block Elements
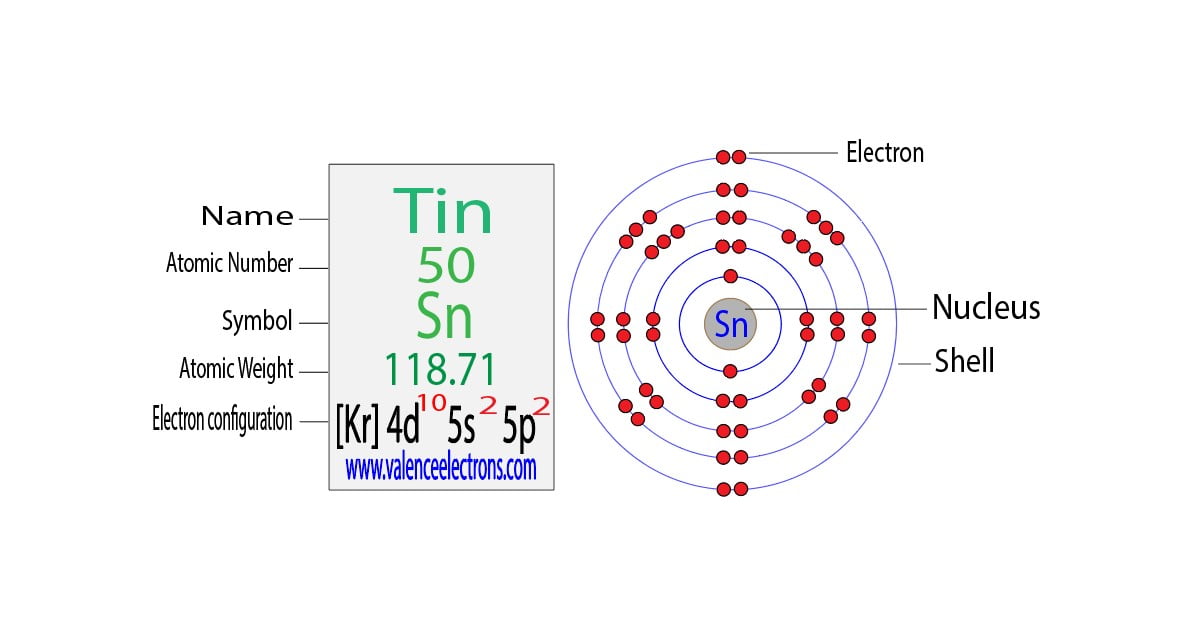
Tin Sn Electron Configuration And Orbital Diagram

Tin Atomic Structure Stock Image C018 3731 Science Photo Library

Orbital Molecules In The New Spinel Gav2o4 Inorganic Chemistry

Mixed Valency And Magnetism In Cyanometallates And Prussian Blue Analogues Philosophical Transactions Of The Royal Society A Mathematical Physical And Engineering Sciences